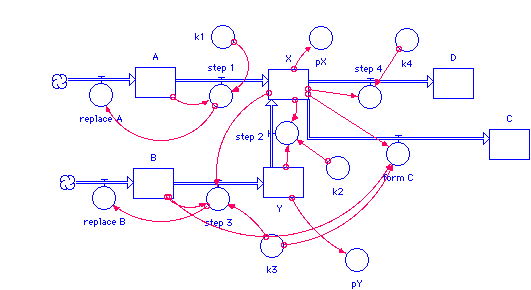
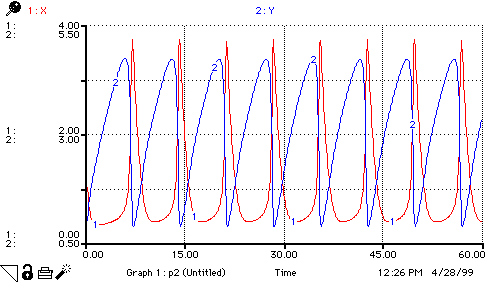
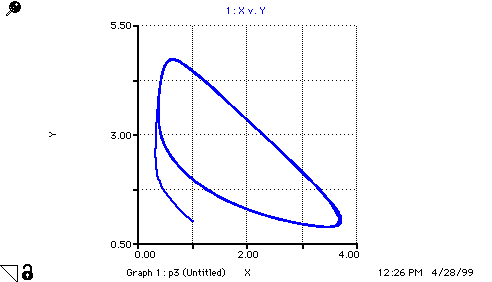
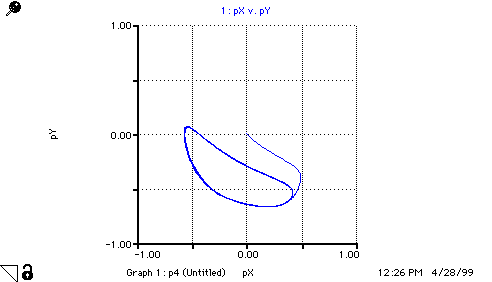
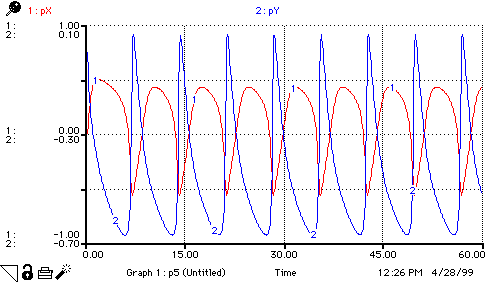
A(t) = A(t - dt) + (replace_A - step_1) * dt INIT A = 1 replace_A = step_1 step_1 = k1*A B(t) = B(t - dt) + (replace_B - step_3) * dt INIT B = 3 replace_B = step_3 step_3 = k3*B*X C(t) = C(t - dt) + (form_C) * dt INIT C = 0 form_C = k3*B*X D(t) = D(t - dt) + (step_4) * dt INIT D = 0 step_4 = k4*X X(t) = X(t - dt) + (step_1 + step_2 - step_4 - form_C) * dt INIT X = 1 step_1 = k1*A step_2 = k2*(X^2)*Y step_4 = k4*X form_C = k3*B*X Y(t) = Y(t - dt) + (step_3 - step_2) * dt INIT Y = 1 step_3 = k3*B*X step_2 = k2*(X^2)*Y k1 = 1 k2 = 1 k3 = 1 k4 = 1 pX = -LOG10(X) pY = -LOG10(Y)