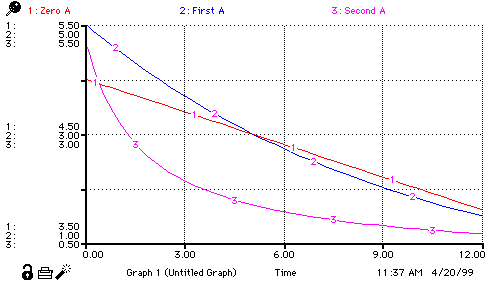
|
|
Vensim Version
|
STELLA Version
|
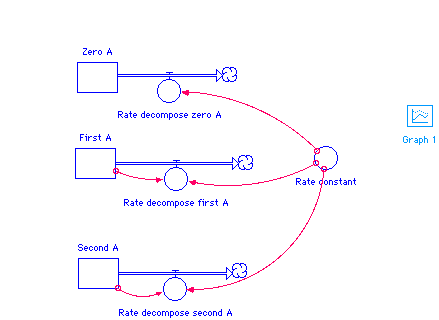
First_A(t) = First_A(t - dt) + (- Rate_decompose_first_A) * dt INIT First_A = 5 OUTFLOWS: Rate_decompose_first_A = First_A*Rate_constant Second_A(t) = Second_A(t - dt) + (- Rate_decompose_second_A) * dt INIT Second_A = 5 OUTFLOWS: Rate_decompose_second_A = Rate_constant*Second_A^2 Zero_A(t) = Zero_A(t - dt) + (- Rate_decompose_zero_A) * dt INIT Zero_A = 5 OUTFLOWS: Rate_decompose_zero_A = Rate_constant Rate_constant = 0.1