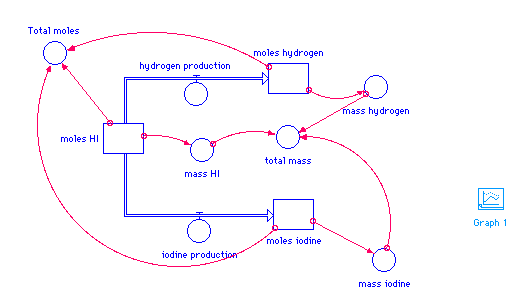
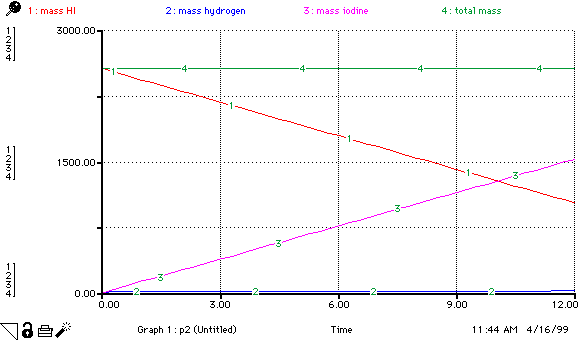
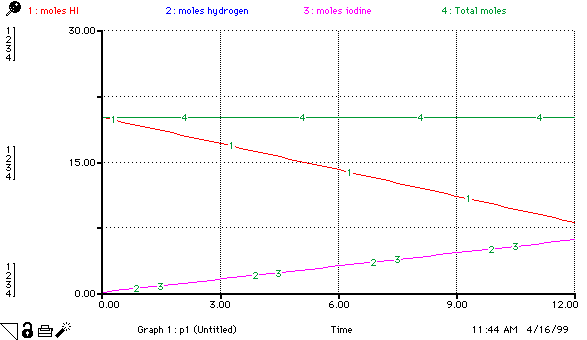
moles_HI(t) = moles_HI(t - dt) + (- hydrogen_production - iodine_production) * dt INIT moles_HI = 20 hydrogen_production = 0.5 iodine_production = .5 moles_hydrogen(t) = moles_hydrogen(t - dt) + (hydrogen_production) * dt INIT moles_hydrogen = 0 hydrogen_production = 0.5 moles_iodine(t) = moles_iodine(t - dt) + (iodine_production) * dt INIT moles_iodine = 0 iodine_production = .5 mass_HI = moles_HI*128 mass_hydrogen = moles_hydrogen*2 mass_iodine = moles_iodine*254 total_mass = mass_HI+mass_hydrogen+mass_iodine Total_moles = moles_hydrogen+moles_iodine+moles_HI