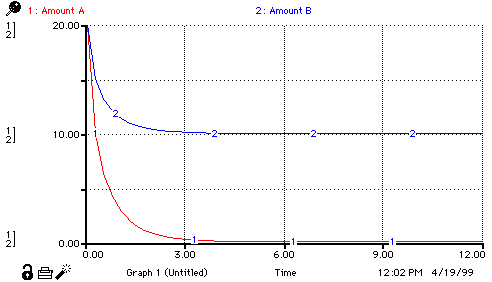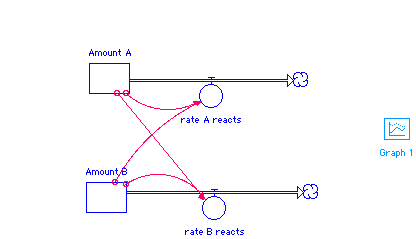
Amount_A(t) = Amount_A(t - dt) + (- rate_A_reacts) * dt INIT Amount_A = 20 OUTFLOWS: rate_A_reacts = .1 * Amount_A * Amount_B Amount_B(t) = Amount_B(t - dt) + (- rate_B_reacts) * dt INIT Amount_B = 20 OUTFLOWS: rate_B_reacts = .05 * Amount_A*Amount_B