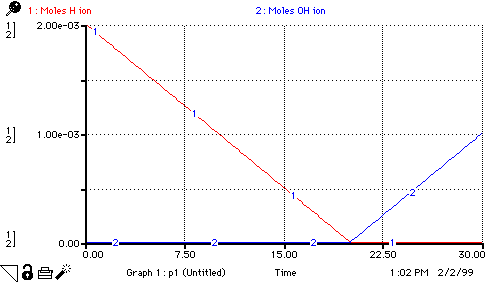
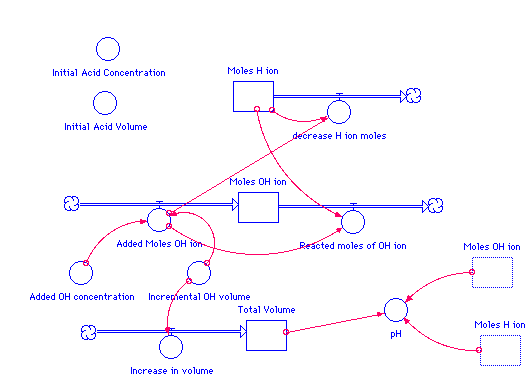
Moles_H_ion(t) = Moles_H_ion(t - dt) + (- decrease_H_ion_moles) * dt
INIT Moles_H_ion = Initial_Acid_Concentration*Initial_Acid_Volume
decrease_H_ion_moles = If Moles_H_ion>0 then Added_Moles_OH_ion else 0
Moles_OH_ion(t) = Moles_OH_ion(t - dt) + (Added_Moles_OH_ion -
Reacted_moles_of_OH_ion) * dt
INIT Moles_OH_ion = 0
Added_Moles_OH_ion = Added_OH_concentration*Incremental_OH_volume
Reacted_moles_of_OH_ion = If Moles_H_ion>0 then Added_Moles_OH_ion else 0
Total_Volume(t) = Total_Volume(t - dt) + (Increase_in_volume) * dt
INIT Total_Volume = Initial_Acid_Volume
Increase_in_volume = Incremental_OH_volume
Added_OH_concentration = 0.1 {M}
Incremental_OH_volume = 0.001 {L}
Initial_Acid_Concentration = 0.1 {M}
Initial_Acid_Volume = 0.020 {L}
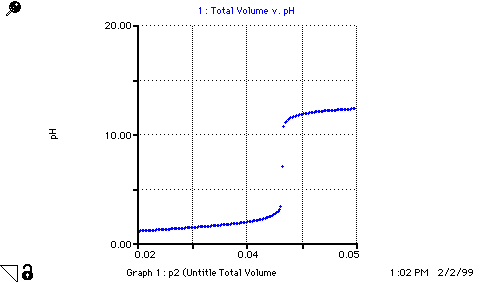
pH = If (Moles_H_ion>0) then (-LOG10(Moles_H_ion/Total_Volume)) else if
(Moles_H_ion=0 AND Moles_OH_ion=0) then 7 else (14
+ LOG10(Moles_OH_ion/Total_Volume))
Time Specs
Range:0-30 , dT = 0.2 , Integration Method =Runge-Kutta 2