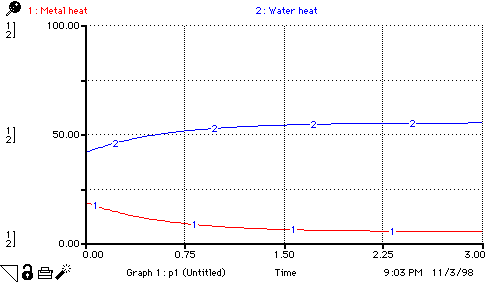
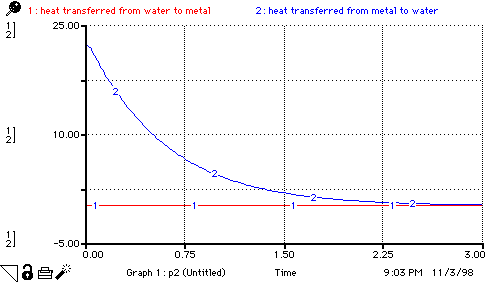
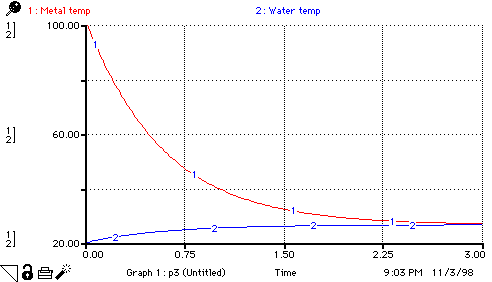
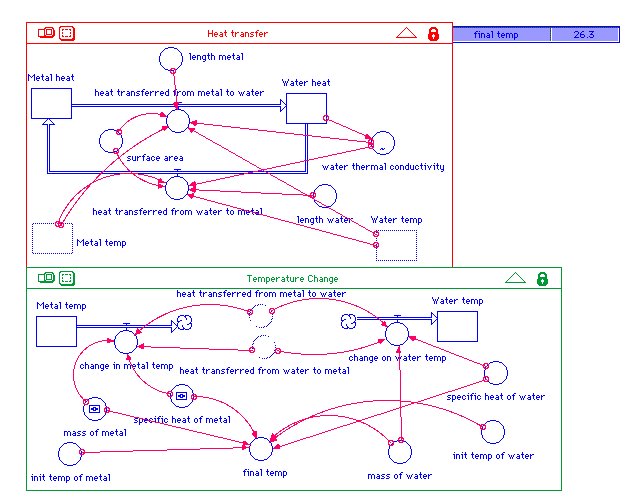
Metal_heat(t) = Metal_heat(t - dt) +
(heat_transferred_from_water_to_metal - heat_transferred_from_metal_to_water)
* dt
INIT Metal_heat = mass_of_metal*specific_heat_of_metal*init_temp_of_metal
heat_transferred_from_water_to_metal =
water_thermal_conductivity*surface_area*(Water_temp-Metal_temp)/length_water
heat_transferred_from_metal_to_water =
water_thermal_conductivity*surface_area*(Metal_temp-Water_temp)/length_metal
Water_heat(t) = Water_heat(t - dt) + (heat_transferred_from_metal_to_water
- heat_transferred_from_water_to_metal) * dt
INIT Water_heat = mass_of_water*specific_heat_of_water*init_temp_of_water
heat_transferred_from_metal_to_water =
water_thermal_conductivity*surface_area*(Metal_temp-Water_temp)/length_metal
heat_transferred_from_water_to_metal =
water_thermal_conductivity*surface_area*(Water_temp-Metal_temp)/length_water
length_metal = .01
length_water = .04
surface_area = .0044
water_thermal_conductivity = GRAPH(Water_heat)
(0.00, 0.561), (10.0, 0.58), (20.0, 0.598), (30.0, 0.615), (40.0, 0.63),
(50.0, 0.643), (60.0, 0.654), (70.0, 0.663), (80.0, 0.67), (90.0, 0.675),
(100, 0.679)
Metal_temp(t) = Metal_temp(t - dt) + (- change_in_metal_temp) * dt
INIT Metal_temp = init_temp_of_metal
change_in_metal_temp =
(heat_transferred_from_metal_to_water-heat_transferred_from_water_to_metal)/
(mass_of_metal*specific_heat_of_metal)
Water_temp(t) = Water_temp(t - dt) + (change_on_water_temp) * dt
INIT Water_temp = init_temp_of_water
change_on_water_temp =
(heat_transferred_from_metal_to_water-heat_transferred_from_water_to_metal)/
(mass_of_water*specific_heat_of_water)
final_temp =
(mass_of_metal*specific_heat_of_metal*init_temp_of_metal+mass_of_water*
specific_heat_of_water*init_temp_of_water)/
(mass_of_water*specific_heat_of_water+mass_of_metal*specific_heat_of_metal)
init_temp_of_metal = 100
init_temp_of_water = 20
mass_of_metal = .2
mass_of_water = .5
specific_heat_of_metal = .907
specific_heat_of_water = 4.18
Time Specs
Range: 0 - 3 , dT = 0.02 , Integration Method = Runge-Kutta 4