Teacher Materials
Student Activity
Learning Goals/Standards
Based on a model from Hans Niedderer and Horst Schecker at the University of Bremen in Bremen, Germany
PC Format
Mac Format
STELLATM software
Charlotte Trout
Williamsport HS
Williamsport, MD
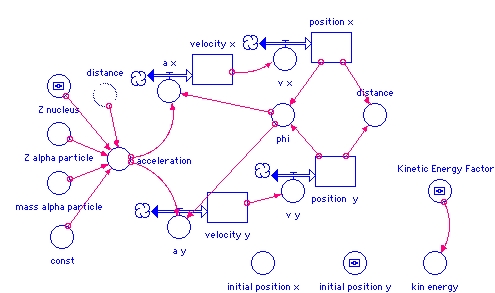
- position_x(t) = position_x(t - dt) + (v_x) * dt
- INIT position_x = initial_position_x
- v_x = velocity_x
- position__y(t) = position__y(t - dt) + (v_y) * dt
- INIT position__y = initial_position_y
- v_y = velocity_y
- velocity_x(t) = velocity_x(t - dt) + (a_x) * dt
- INIT velocity_x = SQRT(2*kin_energy/mass_alpha_particle)
- a_x = acceleration*COS(phi)
- velocity_y(t) = velocity_y(t - dt) + (a_y) * dt
- INIT velocity_y = 0
- a_y = -acceleration*SIN(phi)
- acceleration = -const*Z_alpha_particle*Z_nucleus*
(1.6e-19)^2/distance^2/mass_alpha_particle - const = 1/(4*PI*8.85E-12)
- distance = SQRT(position_x^2+position__y^2)
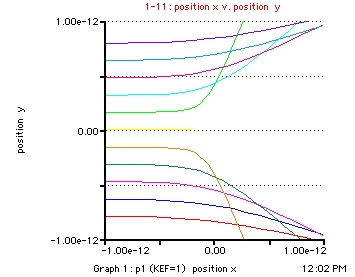
- initial_position_x = -1e-12
- initial_position_y = .01e-12
- Kinetic_Energy_Factor = 1
- kin_energy = Kinetic_Energy_Factor*1e6 {eV} * 1.6e-19 {As}
- mass_alpha_particle = 4*1.66e-27 {kg}
- phi = If position__y>=0 then SQRT((ARCTAN(position__y/position_x))^2) ELSE - SQRT((ARCTAN(position__y/position_x))^2)
- Z_alpha_particle = 2
- Z_nucleus = 79