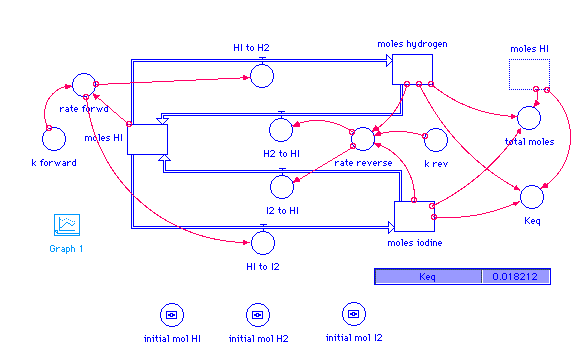
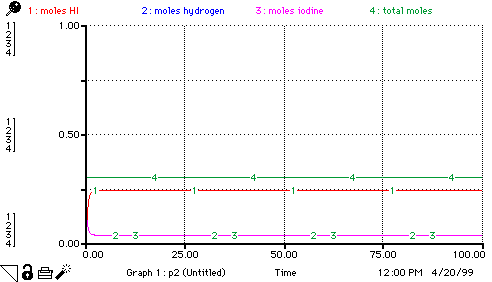
The constants used here are for the reaction at 700K. Here are some other values:
| T(K) | kf | kr | K |
|---|---|---|---|
| 300 | 2.24e-19 | 2.04e-16 | 912 |
| 400 | 2.46e-11 | 6.69e-9 | 371 |
| 500 | 1.66e-6 | 2.14e-4 | 129 |
| 600 | 2.75e-3 | 2.14e-1 | 77.8 |
Students can use these values to explore various aspects of equilibrium. Be forewarned, when changing the rate constants, it may be necessary to change the time specs of the model.
moles_HI(t) = moles_HI(t - dt) + (I2_to_HI + H2_to_HI - HI_to_H2 - HI_to_I2) * dt INIT moles_HI = initial_mol_HI INFLOWS: I2_to_HI = rate_reverse H2_to_HI = rate_reverse OUTFLOWS: HI_to_H2 = rate_forwd HI_to_I2 = rate_forwd moles_hydrogen(t) = moles_hydrogen(t - dt) + (HI_to_H2 - H2_to_HI) * dt INIT moles_hydrogen = initial_mol_H2 INFLOWS: HI_to_H2 = rate_forwd OUTFLOWS: H2_to_HI = rate_reverse moles_iodine(t) = moles_iodine(t - dt) + (HI_to_I2 - I2_to_HI) * dt INIT moles_iodine = initial_mol_I2 INFLOWS: HI_to_I2 = rate_forwd OUTFLOWS: I2_to_HI = rate_reverse initial_mol_H2 = 0.1 initial_mol_HI = 0.1 initial_mol_I2 = 0.1 Keq = moles_hydrogen*moles_iodine/moles_HI^2 k_forward = 0.550 k_rev = 30.2 rate_forwd = k_forward*moles_HI^2 rate_reverse = k_rev*moles_iodine*moles_hydrogen total_moles = moles_HI+moles_hydrogen+moles_iodine