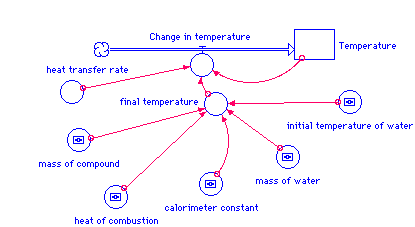
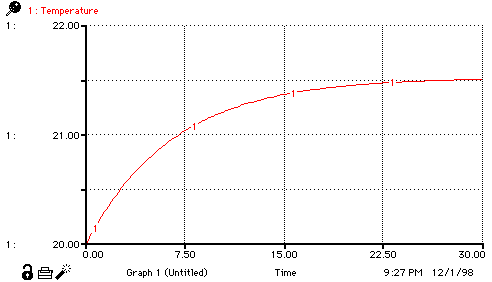
Temperature(t) = Temperature(t - dt) + (Change_in_temperature) * dt
INIT Temperature = initial_temperature_of_water
Change_in_temperature = heat_transfer_rate*(final_temperature-Temperature)
calorimeter_constant = 450 {cal/deg}
final_temperature = (mass_of_compound*heat_of_combustion)/(mass_of_water
+calorimeter_constant)+initial_temperature_of_water
heat_of_combustion = 3718 {cal /g}
heat_transfer_rate = .15
initial_temperature_of_water = 20 {deg C}
mass_of_compound = 1 {g}
mass_of_water = 2000 {g}