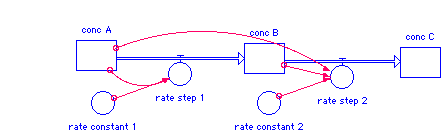
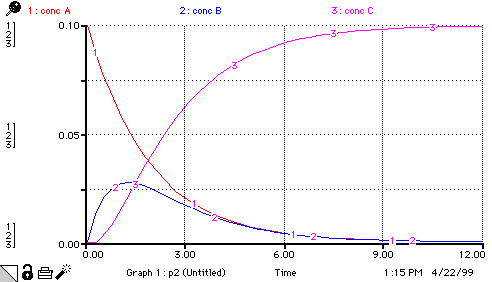
conc_A(t) = conc_A(t - dt) + (- rate_step_1) * dt INIT conc_A = 0.1 rate_step_1 = rate_constant_1*conc_A conc_B(t) = conc_B(t - dt) + (rate_step_1 - rate_step_2) * dt INIT conc_B = 0 rate_step_1 = rate_constant_1*conc_A rate_step_2 = rate_constant_2*conc_B*conc_A conc_C(t) = conc_C(t - dt) + (rate_step_2) * dt INIT conc_C = 0 rate_step_2 = rate_constant_2*conc_B*conc_A rate_constant_1 = 0.5 rate_constant_2 = 1